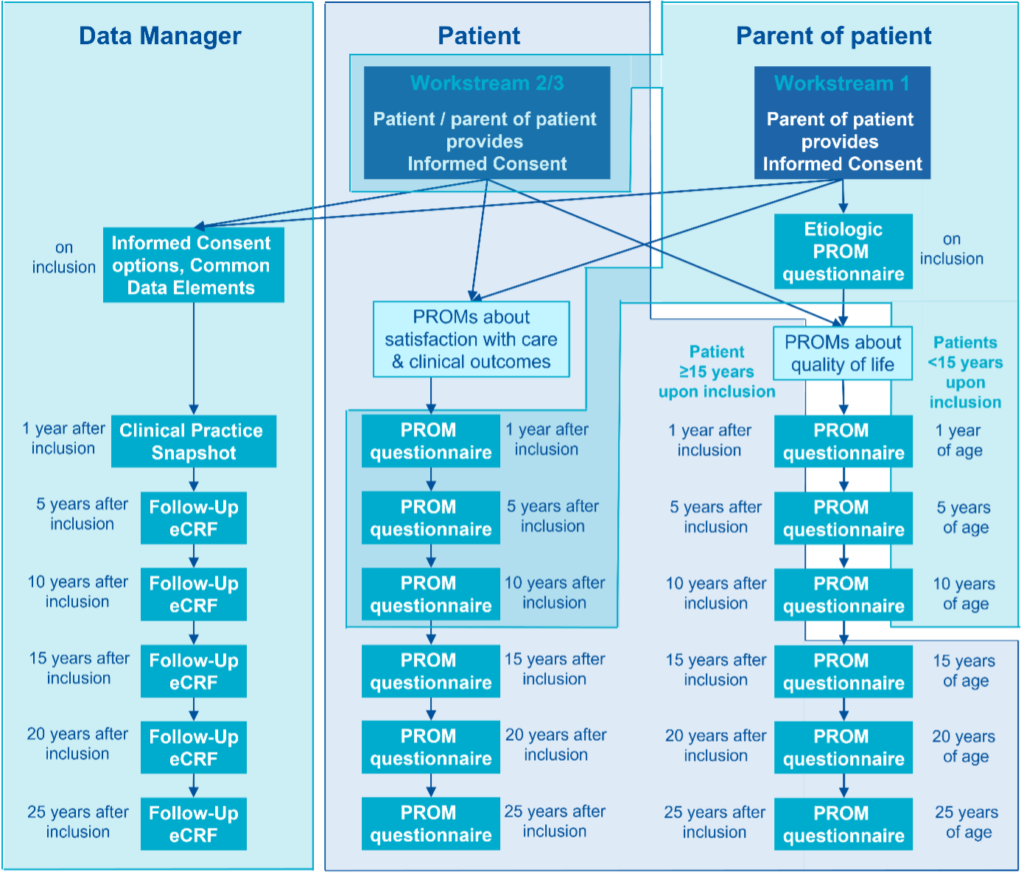
The ERN eUROGEN patient registry is currently being implemented. The aim of this registry is to collect individual data from patients suffering from rare uro-recto-genital diseases or complex conditions. This video explains the concept of the ERN eUROGEN registry for patients. Until now, very limited data has been gathered about disease progression, surgical procedures and treatment outcomes, and the few existing databases are not standardised, fragmenting and scattering the information. Moreover, there is a lack of long-term follow-up of the treatment outcome into adolescence and adulthood, hindering treatment improvement over time. The ERN eUROGEN registry has several objectives: The final ERN eUROGEN registry for all 114 rare uro-recto-genital diseases will benefit patients and their families who go through a diagnosis odyssey, physicians who will learn about rare and complex conditions and get new insights into the best treatment options, and scientists who are looking for patient cohorts for future research and clinical trials. The Principal Investigators at Healthcare Providers (HCPs) involved in ERN eUROGEN as Full Members or Affiliated Partners will make sure that implementation of the ERN eUROGEN registry complies with the rules and regulations of their HCP. This may mean that they need to apply for local approval for the implementation of the ERN eUROGEN registry. Participation in the ERN eUROGEN registry is also open to other HCPs. HCPs will start with retrospective inclusion if they are recognised as a centre of expertise for one or more of the five disease areas for which the Clinical Practice Snapshots were initially developed (1.5 posterior urethral valves, 1.7 anorectal malformations, 1.8 paediatric kidney transplantations, 2.1 complicated & pelvic floor disorders: AMS800 and 3.3 adrenal tumours). These HCPs will start with the prospective inclusion of new patients three months after the start of the retrospective inclusion (which will by then be finalised). HCPs that are not recognised as a centre of expertise for the five disease areas will start prospective inclusion immediately. For prospective inclusion, all patients treated for a rare uro-recto-genital disease or complex condition for which the HCP is recognised as a centre of expertise will be asked for informed consent using the ERN eUROGEN Informed Consent Forms. In the retrospective inclusion, all HCPs will make sure that the last 50 new patients per disease area who visited the HCP before 1 January 2022 are identified and asked for their informed consent to have their data entered in the ERN eUROGEN registry. This will be done by sending the specific Patient Information letter for retrospective inclusion and Informed Consent Forms via regular mail to the patient and/or their parents, depending on the age of the patient. Only patients who provided (or whose parents/legal representatives provided) informed consent will be included in the ERN eUROGEN registry. The ERN eUROGEN platform will be built on the collection of core data set elements and domain-specific elements for specific rare uro-recto-genital diseases. Thus the registry will comprise the following sets of data: Schematic overview of data collection in the ERN eUROGEN registry. Note that the shaded colours of blue indicate who will fill out the information and that part of the Informed Consent forms and PROM questionnaires are filled out by either patients or their parents, depending on the patient’s age. The data in the ERN eUROGEN Registry will be made Findable, Accessible, Interoperable, and Reusable for humans and computers (i.e., FAIR). To achieve this, ERN eUROGEN will work with Bruna Dos Santos Vieira, “FAIRification” Steward, European Joint Program on Rare Diseases (EJP-RD). The ERN eUROGEN registry will be implemented in Castor using a Data Processing Agreement between the Radboudumc and Castor. Only Healthcare Providers who signed the Data Sharing Agreement will obtain access to the online registry. Access of authorized users to the online registry is controlled by the assignment of a secure, individualised password. Scientific Researchers, clinicians, patient organisations, policymakers, companies, etc., are invited to apply for utilisation of the data from the ERN eUROGEN registry. The process of requesting data is described in the Data Access and Sharing Policy. Parmida Anvary, Radboudumc, Nijmegen, The Netherlands: Overview of surgical care for primary closure of Classic Bladder Exstrophy (2025-2027) Parmida Anvary, a PhD student from Radboudumc in Nijmegen, the Netherlands, will conduct a study on classical bladder exstrophy. She will use the data from the ERN eUROGEN registry to create an overview of how the different centres participating in the ERN eUROGEN registry deliver surgical care for primary closure. This will allow her to clearly describe what primary closure entails at each individual centre. She will also create an overview of the different countries in general. She will not only look at the type of primary closure but also focus on the pre-operation care, wound coverage if the closure is delayed, location of the closure surgery (to get an overview of the centralization of CBE care), types of immobilization post-operatively and the effect on bladder dehiscence, wound drainage after the primary closure, and reoperations for complications. Ideally, she wants to be able to conclude which operations have the best outcomes and implement those into a future guideline. Reports comparing clinical practice among different Healthcare Providers have been generated: 2024: 2023: Oomen, L. (2025) Optimizing pediatric kidney transplantation care. A regional, national, and international perspective. Thesis. The PhD thesis of Loes Oomen, former ERN eUROGEN Clinical Data Specialist, shows some research options related to long-term registries. The stakeholders of the ERN eUROGEN Registry are the groups in society who will be positively affected by ERN eUROGEN’s Registry outcome. These are: In managing the ERN eUROGEN registry, different people have different roles as described in the Protocol: Radboudumc is the coordinator of the ERN eUROGEN registry. It has contracted with Castor EDC, a tradename of company Ciwit B.V. to allow the centralized storage of the Study Data. Given the fact that the Provider (the hospital participating in the ERN eUROGEN registry) and Radboudumc jointly developed and determined the purposes and means of the ERN eUROGEN registry, Parties shall be joint Controllers. However, Radboudumc, as the central Party that coordinates the ERN eUROGEN registry, shall be responsible for the storage and the use of the transferred data. Radboudumc shall ensure that appropriate technical and operational measures are in place to safeguard against any unauthorized or unlawful processing of the Study Data and against accidental loss or destruction. Radboudumc shall promptly, and in any event within 48 hours, notify the Provider about any actual or suspected breach of the Data Protection Legislation in relation to any Study Data. The Provider is responsible to obtain and maintain any required ethical approval or similar necessary for the provision of Study Data to the ERN eUROGEN registry. In addition, the Provider is responsible for obtaining any required informed consent from each Data Subject before enrolling the respective Data Subject into the ERN eUROGEN registry and before transferring any data from that Data Subject. To that regard the Provider shall use the patient information and consent form as approved by its competent ethics committee. The consent shall cover inter alia the transfer of pseudonymized data (i.e. removing all sensitive personal data, including but not limited to patient names, initials, and other personally-identifiable information, and leaving only a coded Data Subject number) to Radboudumc and for use within the ERN eUROGEN registry and in scientific research projects approved in accordance with the Data Access and Sharing Policy; Notwithstanding the division of responsibilities set out above, the Parties shall be jointly responsible for the compliance to obligations imposed by the Data Protection Legislation (Article 26 of the GDPR), and the Parties shall cooperate to do all necessary things to enable performance of such compliance obligations. Given the fact that Radboudumc will not have the key to the coded Study Data provided by Provider, The Parties agree that the Provider is primarily responsible for managing Data Subject requests exercising their rights under the GDPR and will comply with article 12 of the GDPR in order to respond to such a request. However, the Parties acknowledge that Data Subjects are allowed to exercise their rights under the GDPR against both Parties. If a Data Subject makes a request to Radboudumc, Radboudumc will notify the Provider about the request without undue delay. Radboudumc will assist Provider in complying with its obligation to provide an answer to Data Subject requests.Registries, Data Management and Analysis
Patient Information Video
Aim
Implementation
Datasets
Data Access
Projects Granted Access
Research Results
Reports
PhD Thesis
Stakeholders
Patients will be the primary target group of the registry, which aims to improve their medical care by stringent performance and outcome monitoring, generating highly relevant prognostic information, and facilitating their access to clinical trials on novel medications and technologies. The registry will include both children and adults, which will offer a unique opportunity to analyse the lifelong evolution of these rare diseases and complex conditions.
The registry will have significant added value for all physicians involved in this field, where there are very few cases to create high-quality standards and EU guidelines. At the same time, the registry could represent a burden due to the time demand of getting used to the platform and filling in the data sets. Crucially, without the collaboration of network HCPs, this registry will not succeed. Therefore, HCPs are probably the most important target group.
The registry will be highly valuable to healthcare administrators on the local, regional, national and EU levels as it will generate novel information on disease epidemiology, management quality, and health outcomes that can be used for cost efficiency analyses.
The registry will be very useful for both the pharmaceutical industry and independent academic researchers in need of information on specific disease populations potentially suitable for involvement in clinical trials, epidemiological studies, and translational research.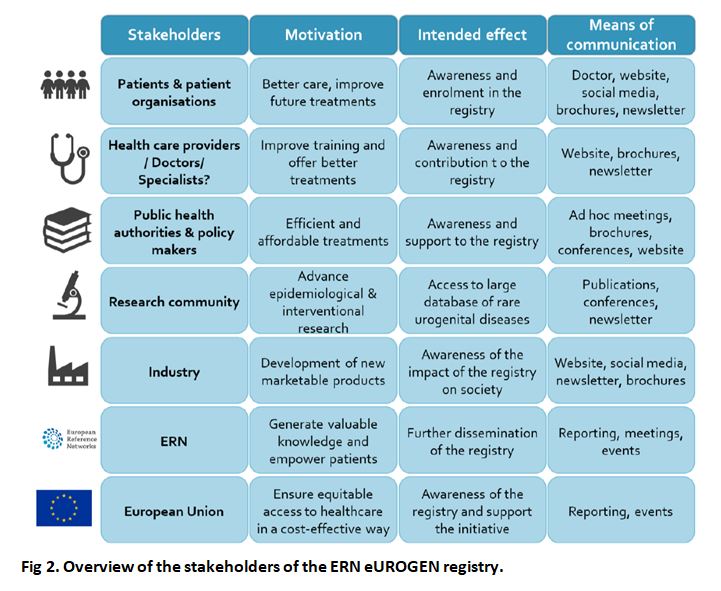
Project Management
GDPR Responsibilities

